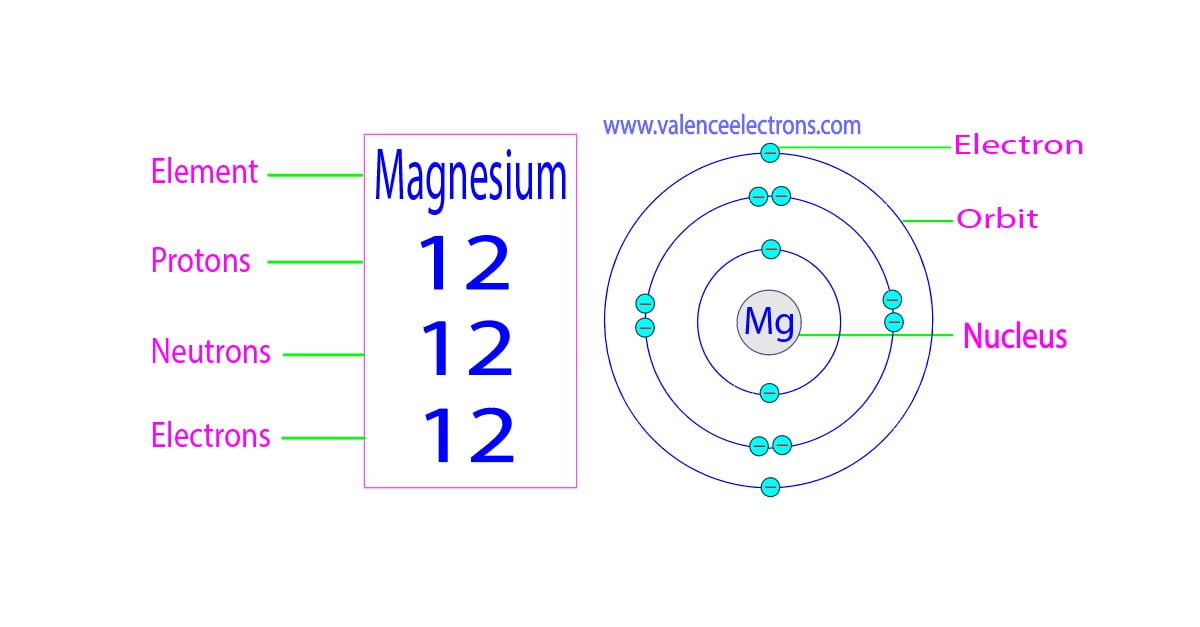
Magnesium Ion Electrons And Protons . In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. The symbol for the ion is mg. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium has two more protons in its nucleus than neon. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. — protons and neutrons in magnesium. magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. It is stable and does not carry. It is an essential ion. Magnesium has two valence electrons in. — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion.
from valenceelectrons.com
It is an essential ion. magnesium has two more protons in its nucleus than neon. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. The symbol for the ion is mg. magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. Magnesium has two valence electrons in. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. It is stable and does not carry. — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion.
How Many Protons,Neutrons and Electrons Does Magnesium Have?
Magnesium Ion Electrons And Protons It is stable and does not carry. magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. — protons and neutrons in magnesium. It is stable and does not carry. magnesium has two more protons in its nucleus than neon. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. Magnesium has two valence electrons in. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. The symbol for the ion is mg. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. It is an essential ion. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Magnesium Ion Electrons And Protons magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. It is stable and does not carry. thus, a magnesium atom will form a cation with two fewer electrons than protons. Magnesium Ion Electrons And Protons.
From mataseluruhdunia202.blogspot.com
Modelo Atomico De Magnesio Animal Magnesium Ion Electrons And Protons In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. It is stable and does not carry. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Ion Electrons And Protons.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Ion Electrons And Protons Magnesium has two valence electrons in. It is an essential ion. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. The symbol for the ion is mg. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. thus, a magnesium atom will. Magnesium Ion Electrons And Protons.
From dxozgzczq.blob.core.windows.net
Magnesium Protons Electrons And Neutrons at Jackie Cope blog Magnesium Ion Electrons And Protons — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons,. Magnesium Ion Electrons And Protons.
From www.numerade.com
SOLVED + 2e 12 protons, 12 electrons 12 protons, 10 electrons Fanion Magnesium Ion Electrons And Protons Magnesium has two valence electrons in. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. magnesium has two more protons in its nucleus than neon. It is stable and does not carry. — protons and neutrons in magnesium. magnesium ion (mg2+) is a positively charged ion formed when. Magnesium Ion Electrons And Protons.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Ion Electrons And Protons The symbol for the ion is mg. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. — protons and neutrons in magnesium. Magnesium has two valence electrons in. . Magnesium Ion Electrons And Protons.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Electrons And Protons The symbol for the ion is mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. magnesium has two more protons in its nucleus than neon. It is stable and does not carry. It is an essential. Magnesium Ion Electrons And Protons.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Ion Electrons And Protons It is an essential ion. The symbol for the ion is mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12. Magnesium Ion Electrons And Protons.
From mungfali.com
Magnesium Ion Electron Configuration Magnesium Ion Electrons And Protons magnesium has two more protons in its nucleus than neon. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. — (a) since mg 2 + is a cation, its name is the name of the element it comes from plus the word ion. It is stable and does not carry.. Magnesium Ion Electrons And Protons.
From www.dreamstime.com
Diagram Representation of the Element Magnesium Stock Vector Magnesium Ion Electrons And Protons magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. It is an essential ion. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom. Magnesium Ion Electrons And Protons.
From valenceelectrons.com
How Many Protons,Neutrons and Electrons Does Magnesium Have? Magnesium Ion Electrons And Protons The symbol for the ion is mg. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. magnesium has two more protons in its. Magnesium Ion Electrons And Protons.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Electrons And Protons magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. The symbol for the ion is mg. thus, a magnesium atom will form a cation with two fewer electrons than. Magnesium Ion Electrons And Protons.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Magnesium Ion Electrons And Protons magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. It is an essential ion. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. magnesium has two more protons in its nucleus than neon. It is stable and does. Magnesium Ion Electrons And Protons.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Electrons And Protons In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. magnesium has two more protons in its nucleus than neon. — (a) since mg 2 + is a cation, its name is the. Magnesium Ion Electrons And Protons.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Magnesium Ion Electrons And Protons thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. The symbol for the ion is mg. It is stable and does not carry. magnesium ion (mg2+) is a positively charged. Magnesium Ion Electrons And Protons.
From www.slideshare.net
The periodic table Magnesium Ion Electrons And Protons Magnesium has two valence electrons in. — protons and neutrons in magnesium. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. magnesium has two more protons in its nucleus than neon. — (a) since mg 2 + is a cation, its name is the name of the element it. Magnesium Ion Electrons And Protons.
From dxohjpizz.blob.core.windows.net
Magnesium Ion Have A Charge at Robert Ezell blog Magnesium Ion Electrons And Protons — protons and neutrons in magnesium. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In total this element has 12 electrons and as the magnesium ion loses two electrons in its valence. a magnesium atom is a neutral particle that consists of 12 protons, 12 neutrons, and 12 electrons. magnesium has two more protons in. Magnesium Ion Electrons And Protons.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Magnesium Ion Electrons And Protons magnesium is the 12th element in the periodic table and has a symbol of mg and atomic number of 12. magnesium ion (mg2+) is a positively charged ion formed when a magnesium atom loses two electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It is stable and does not carry. a magnesium atom is. Magnesium Ion Electrons And Protons.